Dr V. W. Verlekar
Can a Blood Test Tell if Pancreatic Cancer Therapy Works?
IMMUNOLOGY
6/11/20253 min read


Introduction to the Artemis-Delfi Test
In the ongoing battle against pancreatic cancer, researchers have been tirelessly exploring new methods to improve patient outcomes. One of the most promising advancements is the Artemis-Delfi test, an innovative blood test that utilizes artificial intelligence (AI) to evaluate treatment efficacy. This test has the potential to significantly change how healthcare professionals monitor the effectiveness of therapies for this challenging disease.
How Does the Artemis-Delfi Test Work?
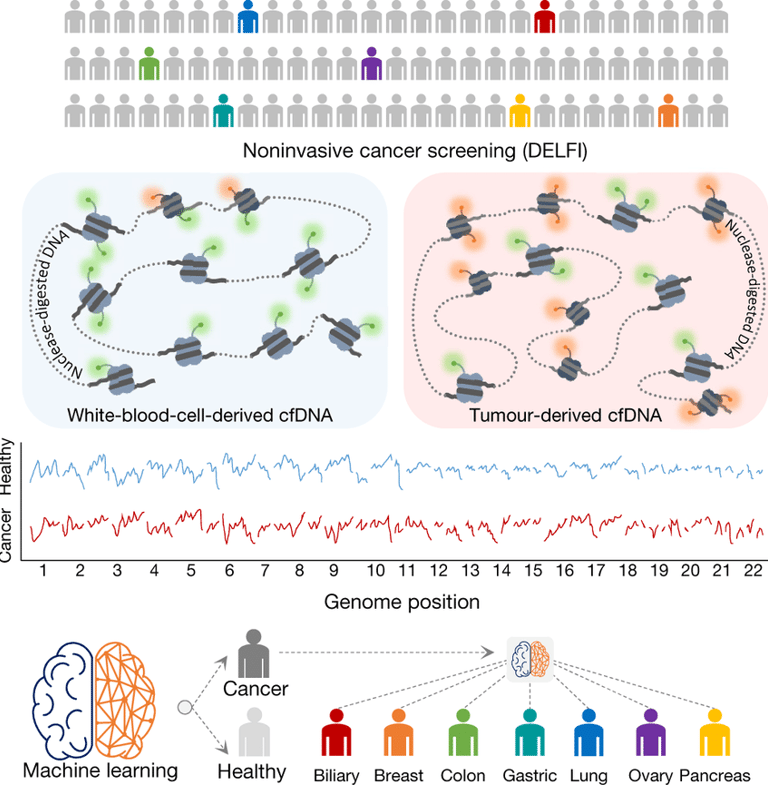
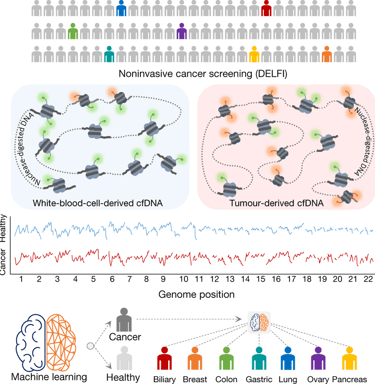
The Artemis-Delfi test focuses on detecting specific DNA fragments shed by pancreatic tumors into the bloodstream. These fragments serve as biomarkers that can indicate how well a patient is responding to treatment. During two large clinical trials assessing immunotherapy for metastatic pancreatic cancer, researchers employed this test, demonstrating its ability to identify patients who exhibited therapeutic responses effectively.
Traditional methods of monitoring treatment progress typically involve imaging studies and other clinical markers. However, these approaches can be time-consuming and sometimes inaccurate. The Artemis-Delfi test, on the other hand, offers a faster and more precise means of tracking therapy effectiveness. Just two months after initiating treatment, the test has shown superior predictive capabilities compared to conventional evaluation methods.
The Advantages of Using AI in Cancer Monitoring
By incorporating artificial intelligence, the Artemis-Delfi blood test enhances the precision and efficiency of patient assessments. AI algorithms can analyze vast amounts of data derived from detected DNA fragments, allowing for a nuanced understanding of a patient's response to therapy. This leads to more informed decisions made by doctors regarding treatment continuance or modification.
Moreover, the implementation of the Artemis-Delfi test could potentially minimize the need for invasive procedures, such as biopsies, that are often uncomfortable for patients. It represents a shift towards non-invasive monitoring, improving the overall patient experience while ensuring timely adjustments to their care plans.
Future Implications for Pancreatic Cancer Treatment
The development of the Artemis-Delfi blood test is a significant step forward in the treatment of pancreatic cancer. Its ability to provide quick and accurate insights into treatment efficacy could lead to better survival rates and quality of life for patients. As researchers continue to explore this technology's capabilities, it holds the promise of becoming a standard tool in oncology.
The integration of advanced techniques like the Artemis-Delfi test into routine clinical practice represents a vital evolution in the fight against pancreatic cancer. As we progress, it remains essential to prioritize innovative solutions that improve patient outcomes and address the challenges posed by this aggressive disease.
Reference : The PACTO Trial
Tracking Progress With Tumor Markers: Beyond ctDNA
CA 19‑9
Historically the main blood tumor marker for pancreatic ductal adenocarcinoma.
Levels correlate with tumor burden; trends downward on effective therapy .
However, limited sensitivity in early disease and false elevations in non-cancerous conditions reduce its utility by itself
Novel Protease-based Assays
One assay, PAC-MANN-1, uses a fluorescent peptide sensor to detect protease activity specific to pancreatic cancer.
In trials, it distinguished pancreatic cancer from benign pancreatic diseases and healthy individuals with high specificity, particularly in early-stage disease when combined with CA 19‑9
🩸 More about Liquid Biopsy
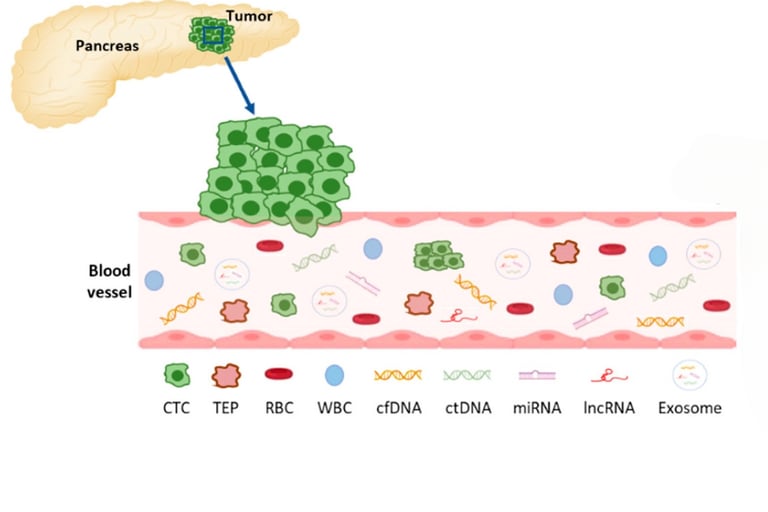
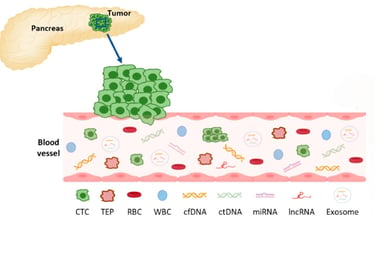
Liquid biopsy analyzes tumor-derived markers—such as circulating tumor DNA (ctDNA), tumor cells, microRNAs, or extracellular vesicles—found in blood. It's non-invasive and can be repeated easily to track tumor changes over time, offering a real-time snapshot of how cancer is responding to therapy
Advances like CAPP‑Seq enable detecting minute ctDNA levels—down to one mutant molecule among 10,000 normal ones—making liquid biopsy a powerful tool to monitor treatment response
Why Liquid Biopsies Are Game-Changers in Monitoring Therapy
Speed: Detect tumor DNA shifts weeks before conventional imaging.
Adaptability: Frequent testing allows real-time adjustments—e.g., switching therapies upon detecting resistance mutations
Precision Oncology: Molecular profiling (e.g., for KRAS, BRCA mutations) helps guide targeted therapies or immunotherapies .
Limitations & Considerations
Not all patients shed detectable ctDNA, especially in early or minimal disease.
Genetic heterogeneity and assay specificity require further refinement.
Clinical protocols for integrating liquid biopsy into routine care are still evolving.